Does Silicon Dioxide Have a High Melting Point
Because the silicon and oxygen atoms are held by strong intermolecular forces. 7 Why is diamond stronger than graphite.

Why Is Carbon Dioxide A Gas While Silicon Dioxide A Solid Quora
Silicon dioxide is mostly obtained by mining including sand mining and purification of quartz.

. Since covalent bonds require more energy to overcome than van der waals SiO2 requires a higher temperature to melt. Silicon Dioxides melting point is 1710 C Whereas Diamond is about 4226 C However it usually does not melt but sublimes and sometimes just convert itself to graphite. Silicon has a very high melting point due to its giant covalent structure.
4 Why do diamond and graphite both have high melting points. 6 Does diamond have a high melting point. Does silicon dioxide have a high or low melting and boiling point.
Even though both carbon and silicon are members of group 14 of periodic table of elements their oxides have different states solid vs. Explain why Silicon Dioxide has a higher melting point than Sulfur Trioxide. Silicon does not melt congruently to give a liquid of the same composition it decomposes at around 2700 0C.
As you heat silicon up the crystal structure changes. This means that it forms thousands of covalent bonds between its silicon and oxygen subunits. That means that at 1 atmosphere pressure carbon dioxide will sublime at a temperature of.
By about covid-19 vaccine locations. Pure silica silicon dioxide when cooled as fused quartz into a glass with no true melting point can be used as a glass fibre for fibreglass. It is very hard with a high melting point.
So it possesses a higher melting point. Silicon IV oxide or SiO2 has a high melting point because it is a solid. Covalent bonds are much stronger than Van der Waals forces and.
Silicon dioxide exists as a giant covalent structure where each Si atom is connected to four oxygen atoms and each oxygen atom is connected to two Si atoms. Melting Point and Freezing Point. This is due to the strength of Si-O-Si binds in the lattice.
4 Why do diamond and graphite both have high melting points. Covalent bonds stronger in SiO2 than in SiCl4 so more energy isneeded to break bonds. 9 What has highest melting point.
5 What is diamond melting point. 10 Does diamond melt or sublimate. Why does silicon dioxide have such a high melting point.
What is silicon dioxide an example of. أنظمة البرامج المحاسبية والإدارية برمجة وتصميم المواقع والمتاجر الإلكترونية والتطبيقات. Due to the tetrahedral structure the melting point of silicon dioxide is very high.
A giant covalent molecule. 3 Why does diamond have a higher melting point than silica. 7 What has highest melting point.
Thus very high energy is required to break the covalent bonds. The strong silicon-oxygen covalent bonds get broken at very high temperatures close to 1700oC. 8 Why is diamond insoluble in water.
495 2724 Views. 11 Which solid has highest melting point. High melting and boiling point.
9 What has highest melting. Covalent bonds so a lot of energy is needed. Silicon dioxide has a high melting point - varying depending on what the particular structure is remember that the structure given is only one of three possible structures but around 1700C.
3 Why does diamond have a higher melting point than silica. 2 Why diamond has very high melting point. Silicon dioxide SiO2 has a macromolecular structure.
متخصصون في تقديم الحلول التقنية والإستشارية للشركات والأنشطة التجارية. 5 What is diamond melting point. 8 Does carbon have a low boiling point.
Sulfur Trioxide has a simple molecular structure meaning it has Van der Waals forces between molecules. Why does silicon dioxide have a high melting point. Quartz is suitable for many purposes while chemical processing is required to make a purer or otherwise more suitable eg.
A lot of energy is needed to break the strong covalent bonds throughout the structure. 43 Votes The bond energy of Si is generally considered to be lower than that of the C-C so a simple explanation is that diamond has a stronger bond. Why does silicon dioxide have a high melting point.
Silicon Dioxide has a macromoleculargiant covalent structure which means it has covalent bonds between all atoms in its structure. It will only melt and liquefy at a specific pressure. 6 Does carbon have a high boiling point.
8 Why is diamond insoluble in water. SiO2 has a high melting point 1700 degrees Celsius. 6 Does diamond have a high melting point.
Also silicon dioxide is very hard and rigid and this is again due to the strong covalent bond between silicon and oxygen. Silicon dioxide has a higher melting point than sodium chloride. What needs to be broken when heating silicon dioxide.
13 Why does carbon dioxide have a. Melting point of carbon dioxide. 1710 C.
12 Why carbon compounds have low boiling and melting point. In comparison carbon dioxide is a gas at room temperature. 9 How do you determine the highest melting point.
7 Why is diamond stronger than graphite. 10 Why is melting point higher than expected.
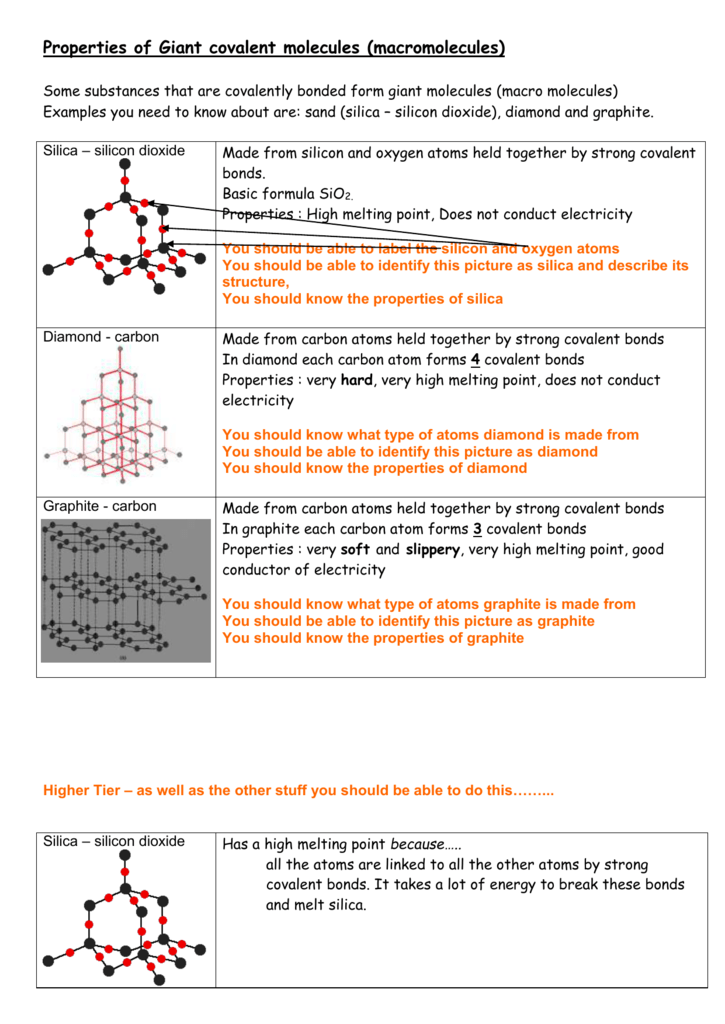
Properties Of Giant Covalent Molecules Macromolecules

4 2 10 Describe The Structure Of And Bonding In Silicon And Silicon Dioxide Youtube

Based On Their Structures Explain Why Sodium Oxide Silicon Dioxide And Carbon Dioxide Have Different Melting Points Here Are 6 Real Student Answers Ppt Download
No comments for "Does Silicon Dioxide Have a High Melting Point"
Post a Comment